NEW YORK – November 25, 2025
Anavex Life Sciences Corp. (“Anavex” or the “Company”) (Nasdaq: AVXL), a clinical-stage biopharmaceutical company focused on developing innovative treatments for Alzheimer’s disease, Parkinson’s disease, schizophrenia, neurodevelopmental, neurodegenerative, and rare diseases, including Rett syndrome and other central nervous system (CNS) disorders, today reported financial results for its fourth quarter of fiscal 2025.
“There are currently an estimated 7.2 million people living with Alzheimer’s disease in the U.S. and 7 million in Europe, respectively. We continue to be focused on orally, targeted upstream medicines, particularly in the context of early Alzheimer’s disease,” said Christopher U. Missling, PhD, President and CEO of Anavex. “Our clinical pipeline positions Anavex to address critical unmet needs in neurodegenerative and neurodevelopmental disorders with convenient and scalable therapeutic options. Oral blarcamesine demonstrated continued clinically meaningful benefit in early-stage Alzheimer’s patients—further validating its therapeutic potential. Following the recent announcement the Company intends to request a re-examination of the CHMP opinion upon its formal adoption, representing our commitment to bring this innovative medicine to patients in need of new treatment options.”
Expected Development Milestones:
- Regulatory and clinical trial update for blarcamesine in early Alzheimer’s disease
- Regulatory and clinical trial update for blarcamesine in Parkinson’s disease
- Regulatory and clinical trial update for blarcamesine in Rett syndrome
- Fragile X development update: Design of Phase 2/3 clinical trial
- Advancing ANAVEX®3-71 towards pivotal clinical studies for the treatment of schizophrenia-related disorders
- Expanding collaborative initiatives and strategic partnership activities
- New scientific findings to be presented at upcoming conferences or publications:
- The direct relationship between cognitive function and reduced brain region atrophy with blarcamesine
- Oral blarcamesine for early symptomatic Alzheimer’s: Robust effect size through Precision Medicine – analysis of the ANAVEX2-73-AD-004 randomized trial
- Newly identified Precision Medicine gene, COL24A1, with >70% prevalence establishes effective treatment of early Alzheimer’s disease with blarcamesine
- Continued long-term benefit from oral blarcamesine compared to delayed-start analysis and natural history studies
Recent Corporate Developments:
- On August 20, 2025, Anavex announced a peer-reviewed publication in Neuroscience Letters, titled “Prevention of memory impairment and hippocampal injury with blarcamesine in an Alzheimer’s disease model,” demonstrating that pre-treatment prevented amyloid beta–induced memory impairment and oxidative injury.
- On August 26, 2025, a peer-reviewed publication in iScience described the precise autophagy mechanism of SIGMAR1 activated by blarcamesine, titled “Conserved LIR-specific interaction of Sigma-1 receptor and GABARAP.”
- On September 30, 2025, Anavex announced a preprint describing the Precision Medicine–defined population in the Phase IIb/III blarcamesine trial, reporting significant clinical and quality-of-life improvements for early Alzheimer’s patients.
- On September 9, 2025, Anavex reported latest findings showing that early Alzheimer’s patients in the Precision Medicine population (“ABCLEAR3”[1]) taking 30 mg oral blarcamesine demonstrated barely detectable decline after 48 weeks.
- On October 2, 2025, Anavex announced positive topline Phase 2 results for ANAVEX®3-71 in schizophrenia (NCT06245213), meeting its primary endpoint with strong safety and encouraging EEG/ERP biomarker trends.
- On October 29, 2025, new data showed continued long-term benefit from oral blarcamesine compared to decline observed in the Alzheimer’s Disease Neuroimaging Initiative (ADNI)[2] control cohort.
- On November 14, 2025, Anavex announced a negative trend vote by the EMA’s CHMP for the blarcamesine MAA. The Company intends to request a re-examination following formal adoption of the opinion.
- On November 19, 2025, Anavex announced it will present at the 44th Annual J.P. Morgan Healthcare Conference on January 14, 2026.
Financial Highlights:
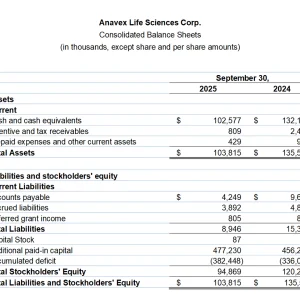
- Cash and cash equivalents of $102.6 million at September 30, 2025, compared to $132.2 million at September 30, 2024. With a current balance over $120 million, the Company anticipates a cash runway of more than 3 years.
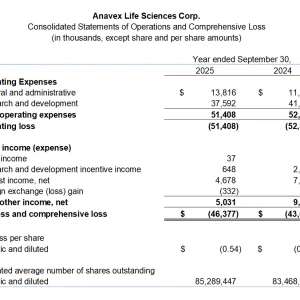
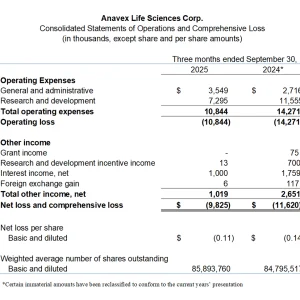
- Research and development expenses for the quarter were $7.3 million, compared to $11.6 million in Q4 2024.
- General and administrative expenses for the quarter were $3.5 million, compared to $2.7 million in Q4 2024.
- Net loss for the quarter was $9.8 million ($0.11 per share), compared to $11.6 million ($0.14 per share) in Q4 2024.
The financial information for the year ended September 30, 2025, should be read in conjunction with the Company’s consolidated financial statements, which will appear on EDGAR (www.sec.gov) and on the Anavex website at www.anavex.com.
Webcast / Conference Call Information:
The live webcast will be available at www.anavex.com.
U.S. dial-in: 1-929-205-6099, Meeting ID: 839 7768 4735, Passcode: 825109.
A replay will remain available for 30 days.
About Anavex Life Sciences Corp.
Further information: www.anavex.com.
Follow Anavex on Twitter, Facebook, Instagram, LinkedIn.
Forward-Looking Statements:
Statements in this press release that are not historical facts are forward-looking statements subject to risks and uncertainties. Actual results may differ materially from those projected. See the Company’s most recent Annual Report on Form 10-K filed with the SEC. The Company undertakes no obligation to update forward-looking statements.
For Further Information:
Anavex Life Sciences Corp.
Research & Business Development — 1-844-689-3939 — info@anavex.com
Investors:
Andrew J. Barwicki — 516-662-9461 — andrew@barwicki.com
[1] ABCLEAR3 = Alzheimer’s Blarcamesine Cognition Efficacy and Resilience gene variants non-carrier population (SIGMAR1 WT / COL24A1 WT).
[2] ADNI = Alzheimer’s Disease Neuroimaging Initiative, a long-term NIH-supported project to develop methods for predicting and tracking Alzheimer’s disease progression.
