ANAVEX3-71-SZ-001 achieved its primary endpoint demonstrating safety and tolerability in adults with schizophrenia
Encouraging trends observed in biomarkers support continued development
NEW YORK – October 2, 2025
Anavex Life Sciences Corp. (“Anavex” or the “Company”) (Nasdaq: AVXL), a clinical-stage biopharmaceutical company developing differentiated therapeutics for the treatment of neurodegenerative, neurodevelopmental, and neuropsychiatric disorders, today announced positive topline results from its placebo-controlled Phase 2 clinical study evaluating ANAVEX®3-71 for the treatment of schizophrenia in adults on stable antipsychotic medication (ANAVEX3-71-SZ-001, NCT06245213).
The study successfully achieved its primary endpoint, demonstrating that ANAVEX®3-71 was safe and well-tolerated. The safety profile was consistent with previous studies of ANAVEX®3-71 in healthy volunteers, with no serious treatment-emergent adverse events (TEAEs) and no severe TEAEs reported in either Part A or Part B of the study.
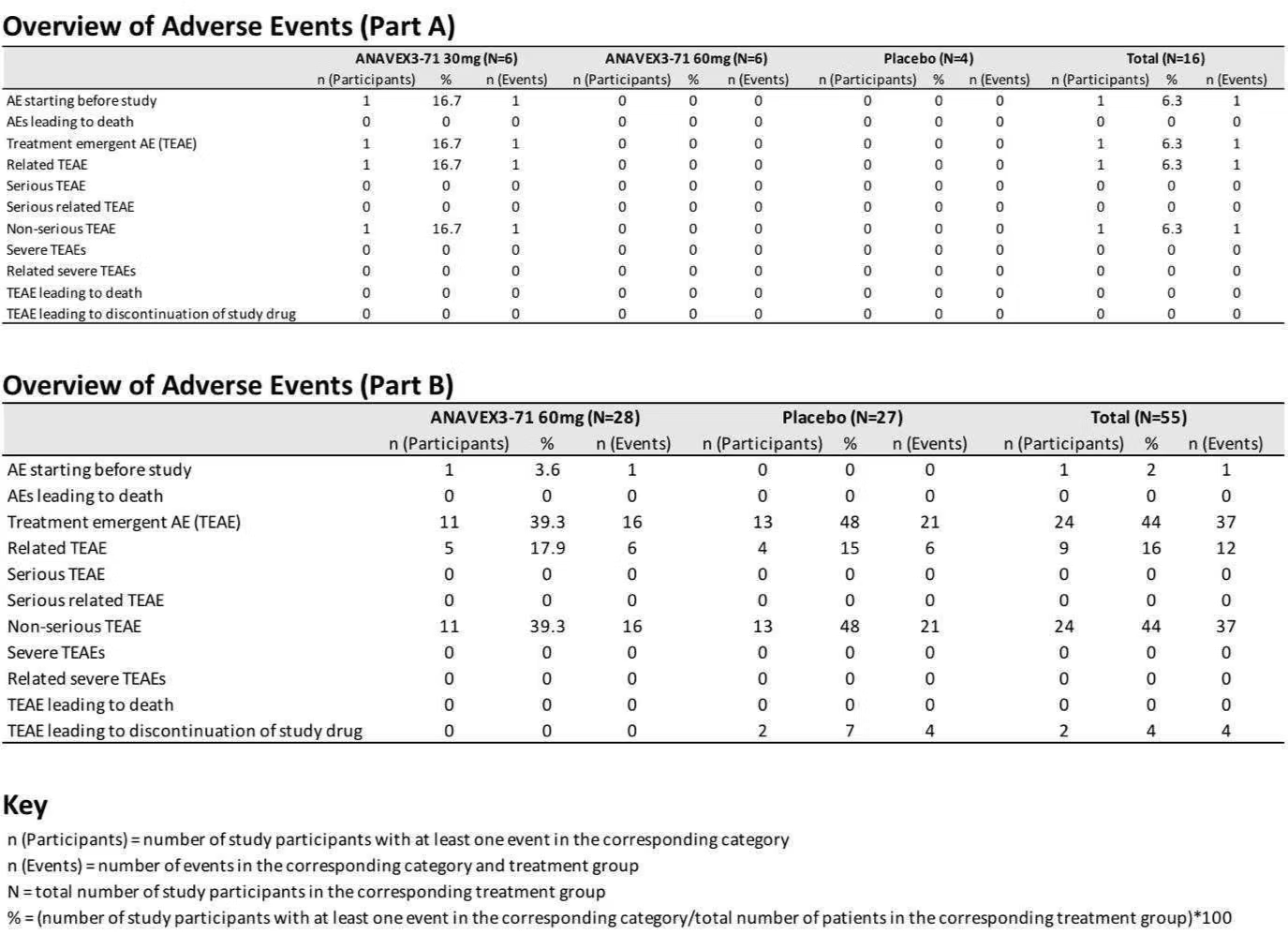
In addition to meeting the primary safety endpoint, secondary and exploratory analyses revealed encouraging trends in several outcome measures. The study demonstrated positive trends in objective electroencephalography (EEG) and event-related potential (ERP) biomarkers of schizophrenia.
Furthermore, neuroinflammatory biomarker assessments showed that glial fibrillary acidic protein (GFAP), a marker of neuroinflammation, was reduced in participants receiving ANAVEX®3-71 compared to placebo. This reduction in neuroinflammatory markers suggests a potential disease-modifying effect that may become more pronounced with longer treatment durations. GFAP is a marker of astrocyte reactivity to neuronal injury and disease [1] with known relevance to both neuropsychiatric [2] and neurodegenerative [3], [4] disorders.
“We are encouraged that our ANAVEX3-71-SZ-001 study aligns with our expectations for safety and tolerability,” said Juan Carlos Lopez-Talavera, MD, PhD, Head of Research and Development of Anavex. “We believe we are well-positioned to advance a competitive candidate into future studies aimed at addressing the ongoing and unmet medical needs of individuals living with schizophrenia and neurodegenerative diseases.”
“We believe this study is a manifestation of Anavex’s continued platform expansion aiming to provide potential beneficial effect for patients with oral compounds,” said Christopher U Missling, PhD, President and Chief Executive Officer of Anavex. “The positive safety profile and encouraging biomarker trends support the continued development of ANAVEX®3-71 as a potential treatment for CNS disorders that could address underlying pathophysiology beyond symptomatic control.”
ANAVEX®3-71 (formerly AF710B) is a dual SIGMAR1 receptor agonist and M1 positive allosteric modulator with agonistic effects [5], [6]. ANAVEX®3-71 has previously been studied in healthy volunteers prior to study ANAVEX3-71-SZ-001 [7], [8]. This novel mechanism of action offers the potential to treat all symptom domains (positive, negative, and cognitive) of schizophrenia without the side effects of standard of care antipsychotics.
About Schizophrenia
Schizophrenia is a persistent and often disabling mental illness impacting how a person thinks, feels, and behaves, and affects nearly 24 million people worldwide, including 2.8 million people in the U.S. It is characterized by three symptom domains: positive symptoms (hallucinations and delusions), negative symptoms (difficulty enjoying life and withdrawal from others), and cognitive impairment (deficits in memory, concentration, and decision-making). In part due to limitations with current treatments, people living with schizophrenia often struggle to maintain employment, live independently, and manage relationships. While current treatments can be effective in managing select symptoms, approximately 34% of people do not respond to therapy [9], with an additional 50–60% experiencing only partial improvement in symptoms or unacceptable side effects [10].
About Anavex Life Sciences Corp.
Anavex Life Sciences Corp. (Nasdaq: AVXL) is a publicly traded biopharmaceutical company dedicated to the development of novel therapeutics for neurodegenerative, neurodevelopmental, and neuropsychiatric disorders, including Alzheimer’s disease, Parkinson’s disease, schizophrenia, Rett syndrome, and other central nervous system (CNS) diseases, pain, and various types of cancer.
Anavex’s lead drug candidate, ANAVEX®2-73 (blarcamesine), has successfully completed a Phase 2a and a Phase 2b/3 clinical trial for Alzheimer’s disease, a Phase 2 proof-of-concept study in Parkinson’s disease dementia, and both a Phase 2 and a Phase 3 study in adult patients and one Phase 2/3 study in pediatric patients with Rett syndrome. ANAVEX®2-73 is an orally available drug candidate designed to restore cellular homeostasis by targeting SIGMAR1 and muscarinic receptors. Preclinical studies demonstrated its potential to halt and/or reverse the course of Alzheimer’s disease. ANAVEX®2-73 also exhibited anticonvulsant, anti-amnesic, neuroprotective, and anti-depressant properties in animal models.
Further information is available at www.anavex.com, and the Company can also be followed on Twitter, Facebook, Instagram, and LinkedIn.
Forward-Looking Statements
Statements in this press release that are not strictly historical in nature are forward-looking statements. These statements are predictions based on current information and expectations and involve risks and uncertainties. Actual events or results may differ materially due to various factors, including risks set forth in the Company’s most recent Annual Report on Form 10-K filed with the SEC. Readers are cautioned not to place undue reliance on forward-looking statements. Anavex undertakes no obligation to update such statements.
For Further Information:
Anavex Life Sciences Corp.
Research & Business Development — Toll-free: 1-844-689-3939 — Email: info@anavex.com
Investors: Andrew J. Barwicki — Tel: 516-662-9461 — Email: andrew@barwicki.com
References
[1] Abdelhak A, Foschi M, Abu-Rumeileh S, et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat Rev Neurol. 2022;18(3):158-172.
[2] Rodrigues-Amorim D, Rivera-Baltanás T, Del Carmen Vallejo-Curto M, et al. Plasma β-III tubulin, neurofilament light chain and glial fibrillary acidic protein in schizophrenia. Sci Rep. 2020.
[3] Yakoub Y, Ashton NJ, Strikwerda-Brown C, et al. Blood biomarker trajectories in preclinical Alzheimer’s. Alzheimers Dement. 2023.
[4] Leipp F, Vialaret J, Mohaupt P, et al. GFAP in Alzheimer’s disease: narrative review. Brain Commun. 2024.
[5] Fisher A, Bezprozvanny I, Wu L, et al. AF710B: M1/σ1 agonist efficacy in AD models. Neurodegener Dis. 2016.
[6] Hall H, Iulita MF, Gubert P, et al. AF710B disease-modifying properties. Alzheimers Dement. 2018.
[7] Fadiran EO, Hammond E, Tran J, et al. Concentration-QTc of ANAVEX3-71. Clin Pharmacol Drug Dev. 2023.
[8] Fadiran EO, Hammond E, Tran J, Missling CU, Ette E. PK/food effect of ANAVEX3-71. Clin Pharmacol Drug Dev. 2024.
[9] Potkin SG, Kane JM, Correll CU, et al. Neurobiology of treatment-resistant schizophrenia. NPJ Schizophr. 2020.
[10] Nucifora FC Jr, Woznica E, Lee BJ, Cascella N, Sawa A. Treatment-resistant schizophrenia: perspectives. Neurobiol Dis. 2019.
