ADAS-Cog13 difference −12.78 (P < 0.0001) with oral blarcamesine treatment compared to ADNI control group at Week 144
77.4 Weeks (17.8 Months) “time saved” with oral blarcamesine compared to ADNI
Restoring impaired autophagy — preceding amyloid-beta and tau
NEW YORK – October 29, 2025
Anavex Life Sciences Corp. (“Anavex” or the “Company”) (Nasdaq: AVXL), a clinical-stage biopharmaceutical company focused on developing innovative treatments for Alzheimer’s disease, Parkinson’s disease, schizophrenia, neurodevelopmental, neurodegenerative, and rare diseases, including Rett syndrome and other central nervous system (CNS) disorders, today announced new findings for blarcamesine, an oral small molecule for the potential treatment of early Alzheimer’s disease.
New data demonstrate continued long-term benefit from oral blarcamesine compared to decline observed in the Alzheimer’s Disease Neuroimaging Initiative (ADNI)[1] control group.
Externally matched control participants from the ADNI database were compared with participants over the 144-week period of ANAVEX®2-73-AD-004 and its ATTENTION-AD (ANAVEX®2-73-AD-EP-004) open-label extension Phase IIb/III trial.[2] For ADAS-Cog13, total score ranges from 0 to 85, with higher scores indicating increased cognitive impairment.
In the intent-to-treat (ITT) population, significantly less cognitive decline was observed for the blarcamesine participants compared to the ADNI control group at 48 weeks, with a significant and clinically meaningful difference in mean change from baseline ADAS-Cog13 total score of −2.68 points (p < 0.0001).[3]
Over the course of the open-label extension study at 96 weeks, these two groups diverged sharply, with statistically significant differences in ADAS-Cog13 mean change of −6.41 points (p < 0.0001). At 144 weeks, the difference increased to −12.78 points (p < 0.0001).
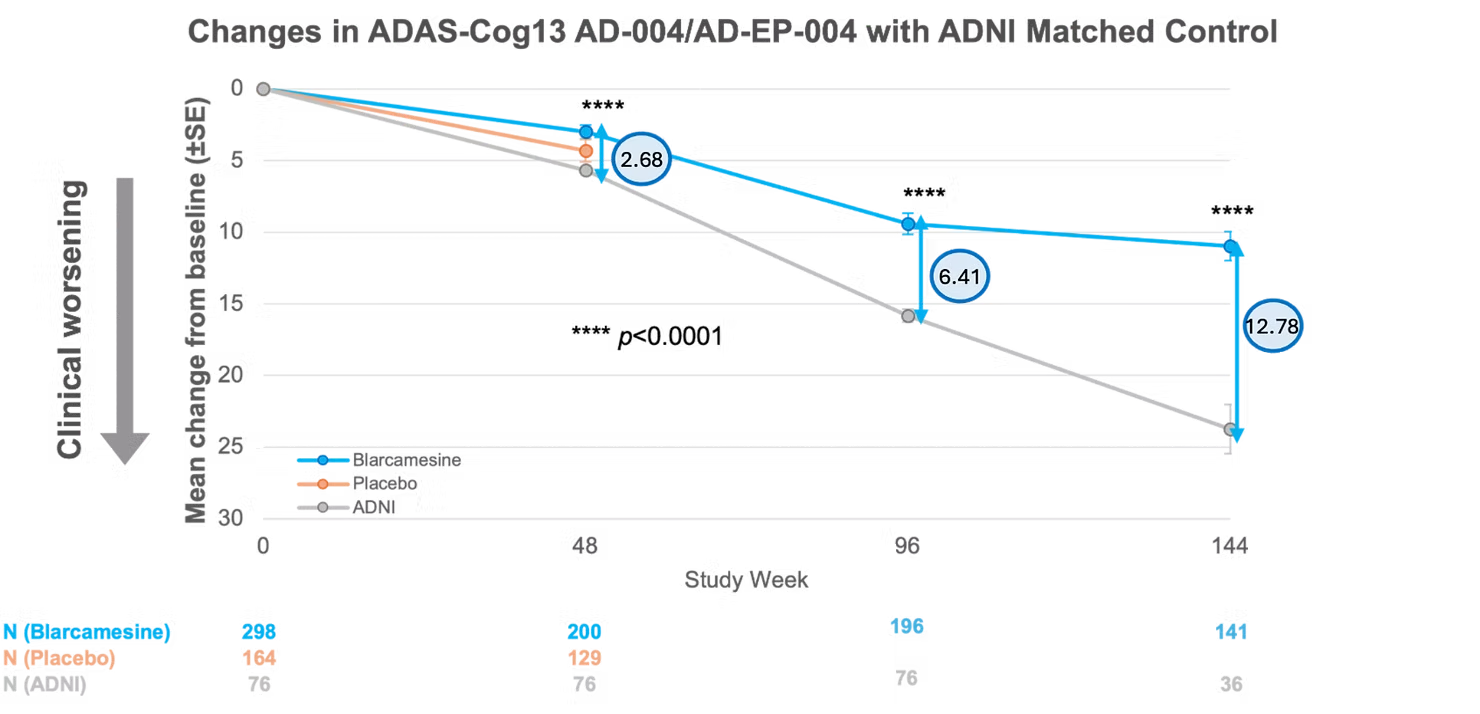
The results provide evidence of the significant beneficial therapeutic effect of blarcamesine, which positively separates from the ADNI control group with duration of treatment.
Alzheimer’s disease “time saved”
In Alzheimer’s disease clinical trials, “time saved” refers to the estimated amount of time a treatment delays disease progression, allowing patients to maintain functionality and independence longer. This measure directly relates to patient daily life.[4][5] Additionally, blarcamesine exhibited a favorable safety profile with no treatment-related deaths.
In the Phase IIb/III early Alzheimer’s disease trial, oral blarcamesine resulted in 77.4 weeks (approximately 17.8 months) of time saved in the ITT population compared to the ADNI control group, underscoring the potential for long-term clinical benefit.
Restoring impaired autophagy
The clinical trial data also confirmed blarcamesine’s upstream mechanism of restoring impaired autophagy early—before amyloid-beta and tau accumulate.
Mechanistic confirmation that blarcamesine restores impaired autophagy through SIGMAR1 activation was previously demonstrated both in vitro and in vivo. Studies showed enhanced autophagic flux in human cells and C. elegans, increased proteostasis capacity, and amelioration of paralysis caused by protein aggregation in C. elegans.[6]
“We remain excited about these enhanced clinically meaningful improvements, accompanied by blarcamesine’s favorable safety profile,” said Juan Carlos Lopez-Talavera, MD, PhD, Head of Research and Development at Anavex. “Convenient once-daily oral dosing may allow us to offer a scalable and patient-friendly option for early Alzheimer’s.”
“We are inspired to advance science across this devastating chronic disease. Alzheimer’s disease requires long-term therapeutic strategy. Blarcamesine’s once-daily oral dosing may lead to greater clinical benefit, as evidenced by significant improvement versus ADNI,” said Christopher U. Missling, PhD, President and CEO of Anavex. “This may help reduce barriers in the healthcare ecosystem and provide broader access to patients.”
Anavex plans to publish and present this new data at international Alzheimer’s disease conferences.
This release discusses investigational uses of an agent in development and is not intended to convey conclusions about efficacy or safety. There is no guarantee that any investigational uses of such product will successfully complete clinical development or gain health authority approval.
About Anavex Life Sciences Corp.
Anavex Life Sciences Corp. (Nasdaq: AVXL) is a publicly traded biopharmaceutical company dedicated to the development of novel therapeutics for neurodegenerative, neurodevelopmental, and neuropsychiatric disorders, including Alzheimer’s disease, Parkinson’s disease, schizophrenia, Rett syndrome, pain, and various cancers. ANAVEX®2-73 (blarcamesine) has completed multiple clinical trials across Alzheimer’s, Parkinson’s disease dementia, and Rett syndrome. It is designed to restore cellular homeostasis via SIGMAR1 and muscarinic receptors.
Further information is available at www.anavex.com. Follow Anavex on Twitter, Facebook, Instagram, and LinkedIn.
Forward-Looking Statements
Statements in this press release that are not strictly historical are forward-looking statements, subject to risks and uncertainties that may cause actual results to differ materially. These risks include those detailed in the Company’s most recent Form 10-K. Readers are cautioned not to place undue reliance on forward-looking statements. Anavex undertakes no obligation to revise or update these statements.
For Further Information:
Anavex Life Sciences Corp.
Research & Business Development — Toll-free: 1-844-689-3939 — Email: info@anavex.com
Investors: Andrew J. Barwicki — Tel: 516-662-9461 — Email: andrew@barwicki.com
References
[1] Alzheimer’s Disease Neuroimaging Initiative (ADNI), NIH, launched 2004.
[2] Observed raw data used; 144-week ADNI control data available.
[3] Muir RT et al., “Minimal clinically important difference in Alzheimer’s disease,” Alzheimers Dement. 2024.
[4] Petersen RC et al., “Expectations and clinical meaningfulness,” Alzheimer’s & Dementia, 2023.
[5] Dickson SP et al., “‘Time Saved’ Calculations,” Journal of Prevention of Alzheimer’s Disease, 2024.
[6] Christ MG et al., “Sigma-1 Receptor Activation Induces Autophagy,” Cells, 2019.
