NEW YORK – August 12, 2025
Anavex Life Sciences Corp. (“Anavex” or the “Company”) (Nasdaq: AVXL), a clinical-stage biopharmaceutical company focused on developing innovative treatments for Alzheimer’s disease, Parkinson’s disease, schizophrenia, neurodevelopmental, neurodegenerative, and rare diseases, including Rett syndrome, and other central nervous system (CNS) disorders, today reported financial results for its third quarter of fiscal 2025.
“Our development of non-invasive, targeted upstream compounds continues to advance, particularly in the context of Alzheimer’s disease,” said Christopher U. Missling, PhD, President and CEO of Anavex. “Clinical feedback highlights the importance of orally administered therapies that are both accessible and effective. At AAIC 2025, we presented open-label extension data for blarcamesine, which demonstrated continued clinically meaningful benefit in early-stage Alzheimer’s patients—further validating its therapeutic potential.”
Recent Highlights:
- On July 31, 2025, Anavex announced the latest findings for blarcamesine, an oral small molecule for the potential treatment of early Alzheimer’s disease. The data were presented by Marwan Noel Sabbagh, MD, Professor of Neurology and Chairman of the Anavex Scientific Advisory Board, at the 2025 Alzheimer’s Association International Conference (AAIC). Blarcamesine-treated patients continue to accrue benefit through up to 4 years, as measured by ADAS-Cog13 and ADCS-ADL. Additional AAIC 2025 presentations featured 48-week Precision Medicine Phase IIb/III ANAVEX®2-73-AD-004 double-blind data, supporting blarcamesine’s upstream mechanism of restoring impaired autophagy.
- From July 27–31, 2025, Anavex participated in the 2025 Alzheimer’s Association International Conference (AAIC) in Toronto, contributing to the global scientific dialogue aimed at advancing dementia research and care.
- In June 2025, a survey of Alzheimer’s disease stakeholders in EU Member States highlighted that oral therapies could significantly “facilitate” patient access and reduce monitoring and administration burdens compared to injectable monoclonal antibodies—potentially supporting broader adoption across healthcare systems.
Financial Highlights:
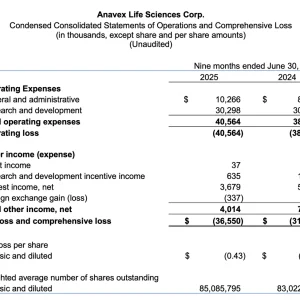
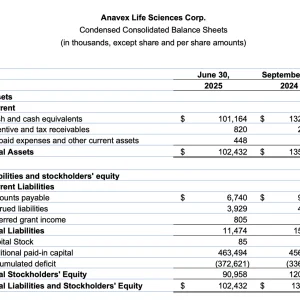
- Cash and cash equivalents of $101.2 million at June 30, 2025, compared to $132.2 million at year-end September 30, 2024. At current adjusted utilization, the Company anticipates a cash runway of more than 3 years.
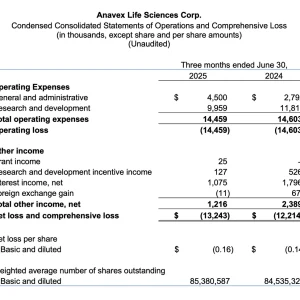
- Research and development expenses were $10.0 million for the quarter, compared to $11.8 million for the same quarter in fiscal 2024.
- General and administrative expenses were $4.5 million for the quarter, compared to $2.8 million in the comparable quarter of fiscal 2024.
- The quarter included an increase in non-cash compensation charges, offset by reduced overall cash operating expenses relative to fiscal 2024.
- Net loss for the quarter was $13.2 million, or $0.16 per share, compared to a net loss of $12.2 million, or $0.14 per share, for the comparable quarter of fiscal 2024.
- Financial information for the quarter ended June 30, 2025, should be read together with the Company’s consolidated financial statements, which will appear on EDGAR at www.sec.gov and on the Anavex website at www.anavex.com.
Webcast / Conference Call Information:
The live webcast of the conference call will be available on Anavex’s website at www.anavex.com.
The conference call may also be accessed by dialing 1-929-205-6099 for U.S. participants and entering Meeting ID# 856 5033 5285 and passcode 014 352. A replay will be available on the Company’s website for up to 30 days.
About Anavex Life Sciences Corp.
Anavex Life Sciences Corp. (Nasdaq: AVXL) is a publicly traded biopharmaceutical company dedicated to the development of novel therapeutics for neurodegenerative, neurodevelopmental, and neuropsychiatric disorders, including Alzheimer’s disease, Parkinson’s disease, schizophrenia, Rett syndrome, and other CNS diseases, as well as pain and certain cancers.
Anavex’s lead drug candidate, ANAVEX®2-73 (blarcamesine), has successfully completed a Phase 2a and a Phase 2b/3 clinical trial for Alzheimer’s disease, a Phase 2 proof-of-concept study in Parkinson’s disease dementia, and both a Phase 2 and Phase 3 study in adult Rett syndrome patients, along with a Phase 2/3 study in pediatric Rett syndrome patients. ANAVEX®2-73 is an orally available compound designed to restore cellular homeostasis by targeting SIGMAR1 and muscarinic receptors. Preclinical studies demonstrated its potential to halt or reverse Alzheimer’s pathology. The compound has also shown anticonvulsant, anti-amnesic, neuroprotective, and antidepressant properties in animal models.
The Michael J. Fox Foundation for Parkinson’s Research previously awarded Anavex a grant fully funding a preclinical study of ANAVEX®2-73 in Parkinson’s disease. ANAVEX®3-71, which targets SIGMAR1 and M1 muscarinic receptors, is also a promising clinical-stage candidate exhibiting disease-modifying activity in transgenic Alzheimer’s models.
Further information is available at www.anavex.com. Connect with Anavex on Twitter, Facebook, Instagram, and LinkedIn.
Forward-Looking Statements
Statements in this press release that are not strictly historical are forward-looking statements based on current information and expectations and involve risks and uncertainties. Actual results may differ materially from projected results due to various factors, including those described in the Company’s most recent Annual Report on Form 10-K filed with the SEC. Readers are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date of this release. Anavex Life Sciences Corp. undertakes no obligation to update or revise these statements to reflect subsequent events or circumstances.
For Further Information:
Anavex Life Sciences Corp.
Research & Business Development
Toll-free: 1-844-689-3939
Email: info@anavex.com
Investors:
Andrew J. Barwicki
Investor Relations
Tel: 516-662-9461
Email: andrew@barwicki.com
